
The arrangement of the atoms needs to be in an ordered, periodic structure for them to diffract the x-ray beams. The science of x-ray crystallography was born.
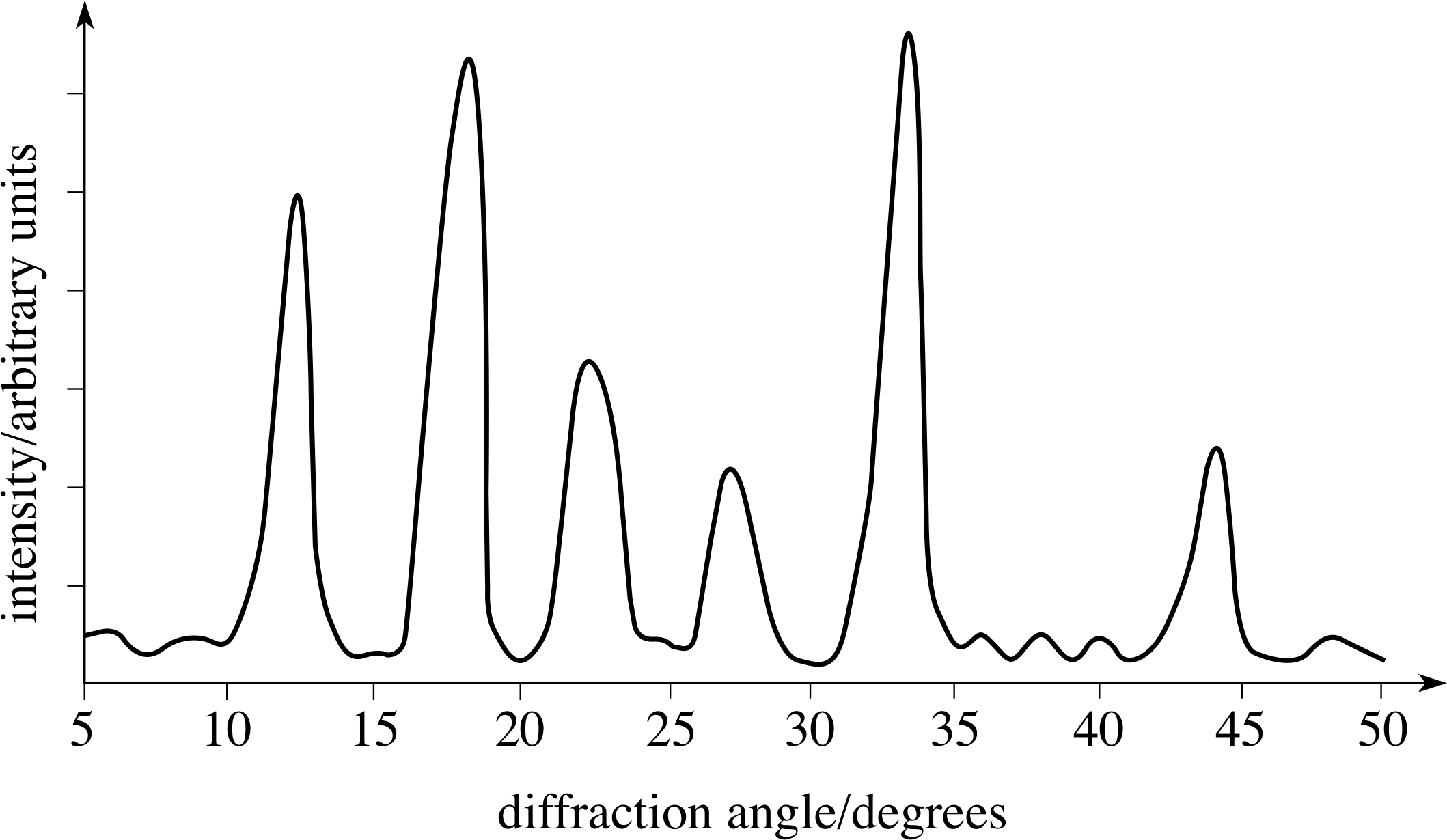
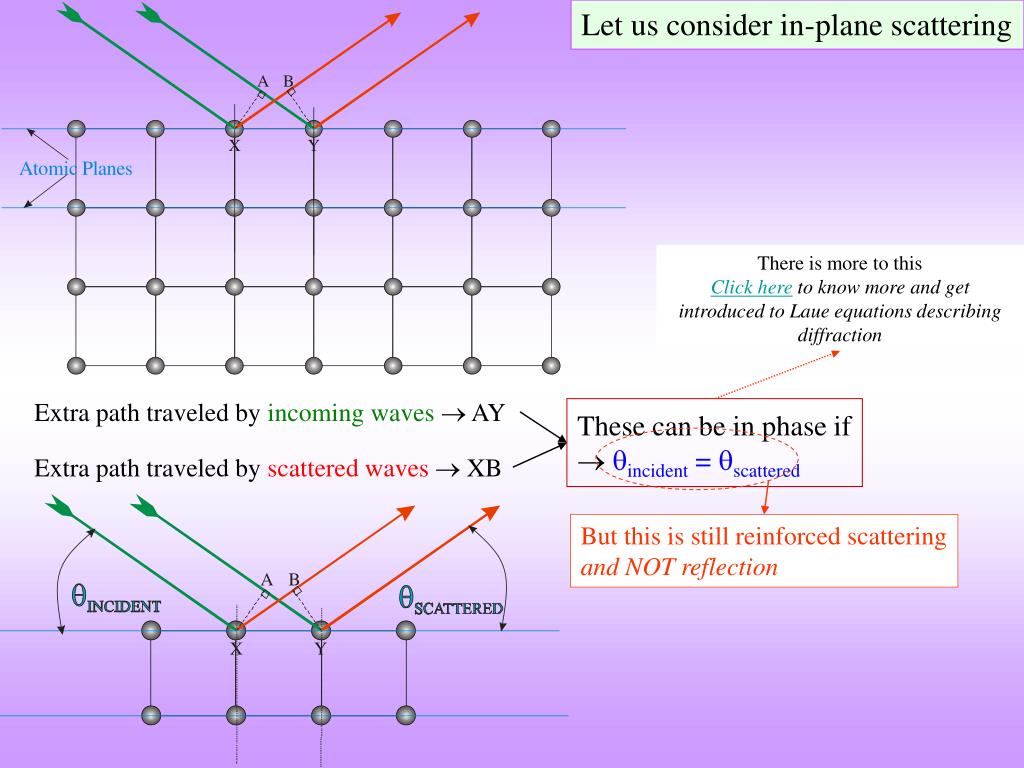
Laue's predictions were confirmed when two researchers: Friedrich and Knipping, successfully photographed the diffraction pattern associated with the x-ray radiation of crystalline \(CuSO_4 \cdot 5H_2O\). His postulate was based on the following assumptions: the atomic lattice of a crystal is periodic, x- rays are electromagnetic radiation, and the interatomic distance of a crystal is on the same order of magnitude as x-ray light. Without having any evidence to support his claim on the periodic arrangements of atoms in a lattice, he further postulated that the crystalline structure could be used to diffract x-rays, much like a grating in an infrared spectrometer can diffract infrared light. In 1912, Max von Laue, at the University of Munich in Germany, postulated that atoms in a crystal lattice had a regular, periodic structure with interatomic distances on the order of 1 Å. Diffraction and measurement of such small wavelengths would require a grating with spacing on the same order of magnitude as the light. If the wave idea was correct, researchers knew that the wavelength of this light would need to be on the order of 1 Angstrom (Å) (10 -8 cm). The nature of x- rays, whether they were particles or electromagnetic radiation, was a topic of debate until 1912.
In 1895, Wilhelm Rontgen discovered x- rays.


 0 kommentar(er)
0 kommentar(er)
